The EURO-WABB Project is a collaboration of doctors, scientists and patient support groups from all over Europe. Within the EU Health Programme 2008-2013 and its call for promoting health through the creation of new registers for rare diseases, EURO-WABB is supported by The EU Directorate General for Health and Consumers (DG-SANCO) via its Executive Agency for Health and Consumers. The overall aim is for this register to be a key instrument to increase knowledge on these rare diseases, improve the lives of affected people through better management, and to develop clinical research.
Wolfram, Alström, Bardet-Biedl (WABB) and other Rare Diabetes Syndromes
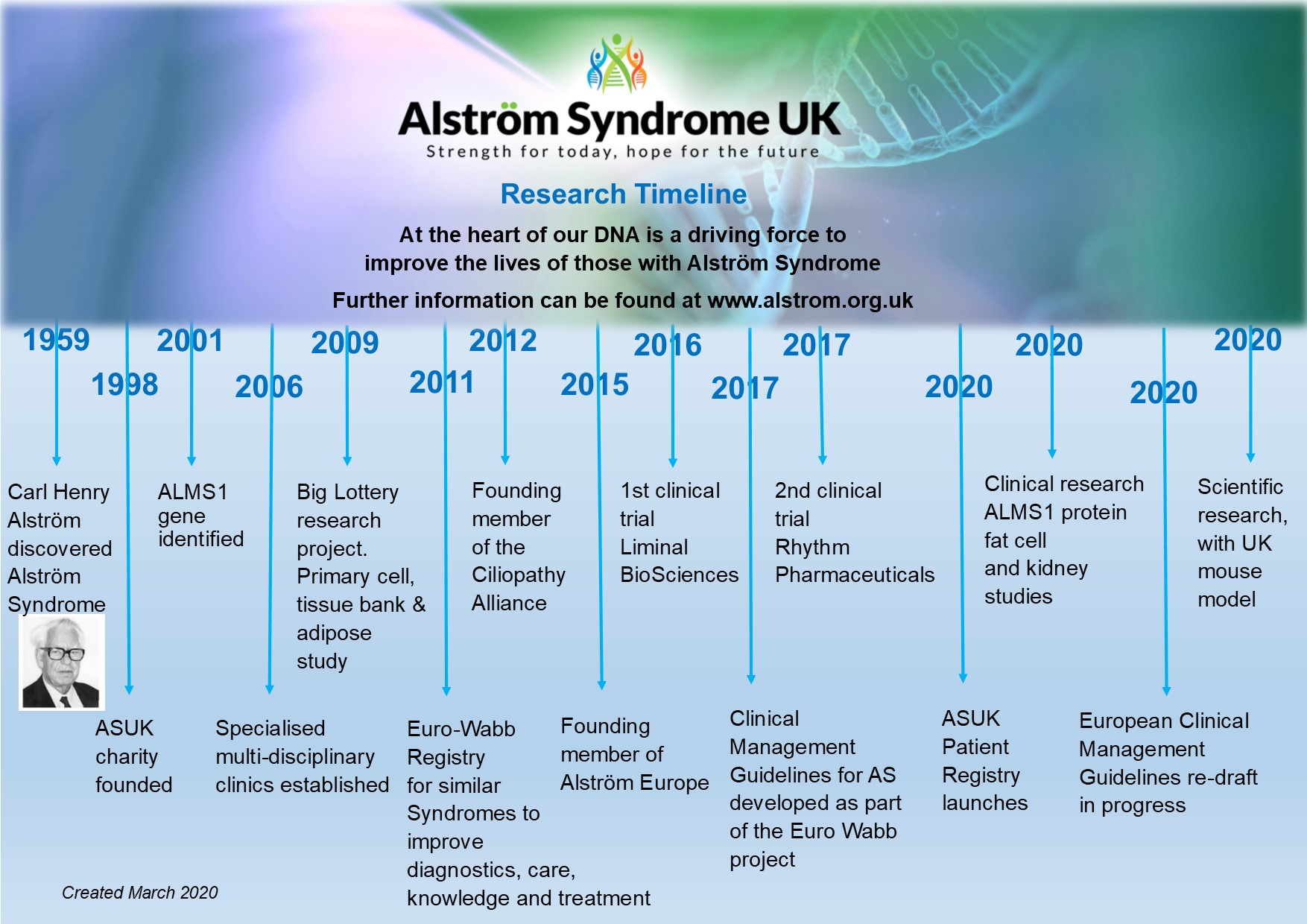
WABB syndromes constitute a group of rare, heritable disorders linked by intolerance of the body to glucose. Each of these syndromes affects other parts of the body, including hearing and vision. This Register is mainly directed towards Wolfram syndrome, Alström syndrome and Bardet-Biedl syndrome; however its scope includes some other rarer syndromes including Wolcott-Rallison syndrome and Thiamine-responsive mebaloblastic anaemia, deafness and diabetes syndrome. Long term studies on these syndromes are desperately needed to understand their natural history, relate genetic diagnosis to predicting outcomes, to establish a basis for evidence-based management, and to develop new treatments.
Structures
The project combines unique strengths by linking a European patient-based data collection registry (work package (WP) 6) with the development of widespread availability of genetic testing across the EU (WP-5), and the development of a core dataset of information and evidence based diagnosis and management pathways (WP-4). WP-1 focuses on coordination of the project, WP-2 on dissemination to patients, health professionals and health policy-makers, and WP-3 on evaluation of the project.
You can find out more about the project via their website HERE





 ASUK is pleased to announce that we are working in partnership with the Canadian Pharmaceutical Company ProMetic Bio therapeutics Ltd and the specialist clinical team at the Queen Elizabeth Hospital, Birmingham to support the first clinical trial for people affected by Alström Syndrome in the UK. The primary aim of the trial is to
ASUK is pleased to announce that we are working in partnership with the Canadian Pharmaceutical Company ProMetic Bio therapeutics Ltd and the specialist clinical team at the Queen Elizabeth Hospital, Birmingham to support the first clinical trial for people affected by Alström Syndrome in the UK. The primary aim of the trial is to
